
Every year, more than 5.8 billion prescription drug packages move through the U.S. supply chain-from factories to warehouses, to pharmacies, and finally to patients. Behind this massive flow is a hidden battle: protecting real medicine from fake. Counterfeit drugs aren’t just a global myth. They’re a real threat. And the system built to stop them is more complex, more technical, and more critical than most people realize.
The System That Keeps Fake Drugs Off the Shelf
The U.S. doesn’t rely on luck or inspections alone to stop counterfeit drugs. It uses a legal and technological framework called the Drug Supply Chain Security Act (DSCSA). Signed into law in 2013, this isn’t just a guideline-it’s a mandatory, decade-long rollout that now requires every prescription drug package to carry a digital fingerprint. That fingerprint is a 2D barcode called a Unique Product Identifier (UPI), which includes the National Drug Code (NDC), a unique serial number, the lot number, and the expiration date. This isn’t just a label. It’s a digital passport for every pill, vial, or syringe.By November 2023, every manufacturer, wholesaler, and pharmacy had to start exchanging this data electronically. No more paper invoices. No more handwritten logs. Every time a drug changes hands, the system records it. If a package turns up suspicious, regulators and companies can trace it back to its exact origin in minutes, not days.
How the Tracking Works: Serialization, Verification, and Authorized Partners
There are four pillars holding up this system. First: serialization. Every package gets a unique code. In the U.S., that’s a 20-character alphanumeric string. That means over 1.2 million unique codes are generated every single day. The system doesn’t just assign them-it verifies them in real time.Second: verification. If a pharmacy scans a bottle and the serial number doesn’t match what the manufacturer says it should be, the system flags it. That’s not a glitch-it’s a red flag. In 2022, this process stopped around 12,000 suspect products from reaching patients. That’s 12,000 chances to avoid a dangerous fake.
Third: authorized trading partners. You can’t just sell drugs to anyone. Every company in the chain-manufacturer, distributor, pharmacy-must be verified by the FDA’s ATP Verification Router Service. This system checks if a company is legally allowed to handle drugs. In Q3 2023, it handled over 50,000 verification requests daily with a 99.8% success rate. If a supplier isn’t on the list, the system blocks the transaction.
Fourth: electronic data exchange. All this information flows through a standard called EPCIS, developed by GS1. It’s the same system used in global logistics for electronics and groceries. But here, it’s saving lives. Over 15 million transactions are processed daily with 99.95% accuracy. If a batch of insulin is recalled, the system can pinpoint every pharmacy that received it within hours.
Real-World Impact: When the System Worked
This isn’t theoretical. In 2022, during the infant formula crisis, the DSCSA system proved its value. When contaminated batches were identified, the FDA used the traceability data to pull affected products from shelves in 72 hours. Before DSCSA, that process took an average of 14 days. That difference meant fewer babies were exposed to harmful substances.Merck’s global serialization team reported that after upgrading to EPCIS 2.0, their verification time dropped from 15 minutes to just 47 seconds. That’s not just faster-it’s safer. Fewer delays mean fewer chances for counterfeit drugs to slip through.
Independent pharmacies have seen results too. One pharmacist on Reddit shared that his ATP verification system caught three fake product offers from unauthorized suppliers in a single month. Those weren’t just rejected orders-they were potential poisonings prevented.

The Gaps: Where the System Still Falls Short
Despite its strengths, the system isn’t perfect. One big weakness: repackaged drugs. When a hospital or pharmacy takes a bulk bottle of pills and puts them into smaller blister packs, the original barcode is destroyed. The new pack gets a new label, but the chain of custody gets fuzzy. That’s a known loophole.Another issue: international gaps. The U.S. uses DSCSA. The European Union uses the Falsified Medicines Directive (FMD). They’re similar in goal but different in design. The EU requires all prescription drugs to have a tamper-proof seal and a 20-digit numeric code. The U.S. allows alphanumeric codes. The EU centralizes verification through national databases. The U.S. uses decentralized data exchange. For companies that sell drugs in both markets, that means running two separate systems. According to PwC, this doubles compliance costs for global firms.
Small pharmacies are struggling. A 2023 survey found that 63% of independent pharmacies with fewer than 10 employees couldn’t meet the 2023 electronic data exchange deadline. One owner said DSCSA compliance cost him $18,500 a year-3.2% of his net profit. For a small business, that’s not just an expense-it’s a survival issue.
Technology and Human Error: The Hidden Risks
Even with perfect systems, humans make mistakes. In 2022, FDA field testing found that 12.7% of barcodes were unreadable due to poor printing, smudging, or packaging design. That means one in eight packages couldn’t be scanned properly. If a system can’t read the code, it can’t verify the drug. That’s a vulnerability.And then there’s cybersecurity. In early 2023, the Change Healthcare cyberattack shut down verification systems for 72 hours. That affected 35% of U.S. pharmacies. For those days, pharmacists couldn’t confirm if a drug was real or fake. They had to rely on manual checks-slower, less reliable, and riskier.
False positives are another problem. The ATP system sometimes flags legitimate suppliers as unauthorized. One pharmacy tech reported an 8.3% false positive rate. That means nearly one in 12 verification attempts is wrong. It creates delays, frustration, and extra work.
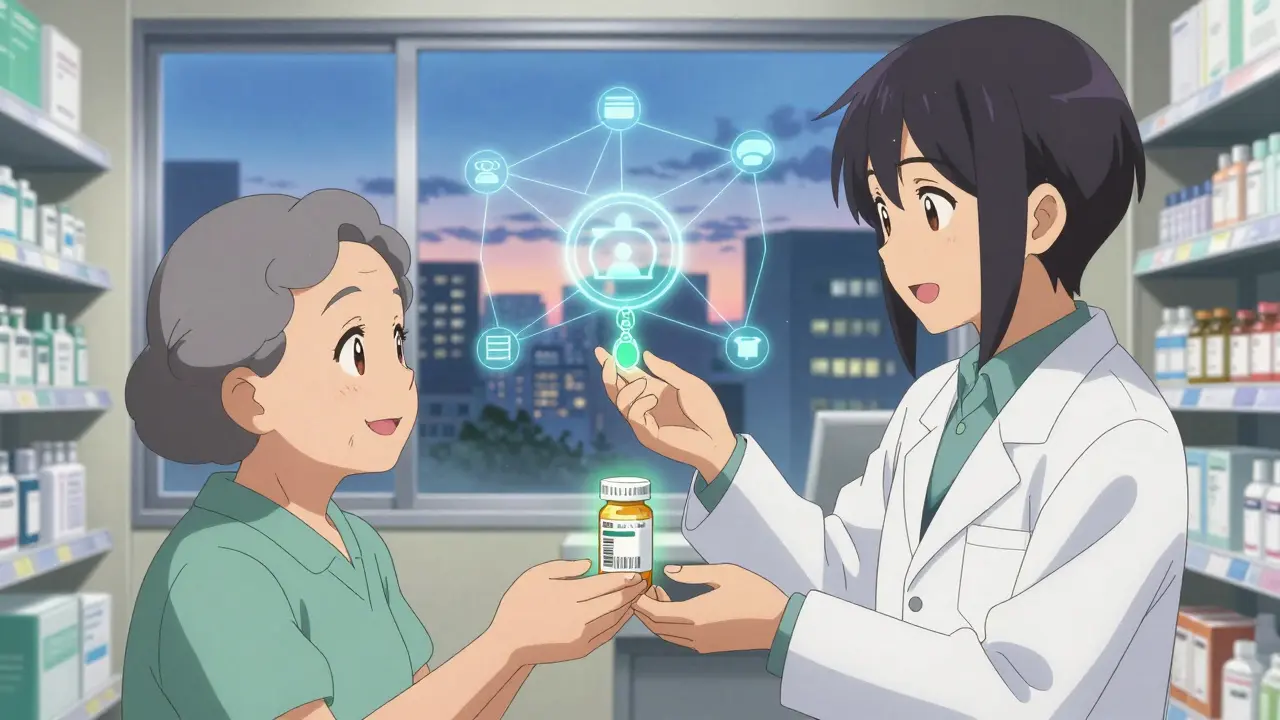
What’s Next: The Road to 2027 and Beyond
The DSCSA isn’t done. By November 2027, every single transaction must be fully electronic and interoperable. That means no more paper, no more incompatible systems. Every player-from the smallest clinic to the biggest manufacturer-must talk to each other in the same digital language.Right now, 47 different software platforms are in use across the U.S. That’s chaos. The FDA’s 2024 Interoperability Pilot Program is testing whether 12 major companies can connect their systems to achieve 99.9% accuracy. If it works, it could set the standard for the entire industry.
Looking ahead, companies are testing AI to detect anomalies in shipping patterns. Some are using IoT sensors to track temperature and humidity in cold-chain drugs like insulin and vaccines. A few are even experimenting with blockchain, though it’s still early. The goal isn’t just to stop counterfeits-it’s to predict them.
McKinsey projects that by 2030, this infrastructure will evolve into a predictive analytics platform. It won’t just react to fake drugs-it’ll spot patterns that suggest where a counterfeit is likely to appear next. That could reduce counterfeit incidents by 95% and save the industry $8.7 billion a year in losses and recalls.
What Patients Should Know
You don’t need to understand EPCIS or serialization to stay safe. But you should know this: if you buy prescription drugs from a licensed pharmacy in the U.S., the chances of getting a fake are extremely low. The system works. It’s not perfect, but it’s the most advanced drug safety network in the world.Don’t buy pills from websites that don’t require a prescription. Don’t trust social media sellers or overseas pharmacies that claim to offer “discounted” brand drugs. The DSCSA protects the legal supply chain-but it can’t reach illegal ones.
If something looks off-different color, smell, or shape-talk to your pharmacist. They’re trained to spot problems. And if you’re ever unsure, call the FDA’s MedWatch hotline. Your vigilance, combined with the system, is what keeps medicine safe.
What is the DSCSA and why does it matter for drug safety?
The Drug Supply Chain Security Act (DSCSA) is a U.S. federal law passed in 2013 to build a secure, electronic system for tracking prescription drugs from manufacturer to patient. It matters because it prevents counterfeit, stolen, or contaminated drugs from entering the legal supply chain. Before DSCSA, fake drugs could circulate for months undetected. Now, if a problem arises, the system can trace and remove affected products in hours, not weeks.
How do I know if my prescription drug is real?
If you get your prescription from a licensed U.S. pharmacy, you can be confident it’s legitimate. The DSCSA system ensures every package is tracked and verified. You don’t need to check barcodes yourself-pharmacists do that automatically when they scan the drug. If you buy drugs online without a prescription or from a foreign website, you’re bypassing this protection and putting yourself at risk.
Are generic drugs more likely to be counterfeit?
No. Counterfeiters target high-demand, high-price drugs regardless of whether they’re brand-name or generic. But because generics are often cheaper and sold in bulk, they’re more attractive to illegal suppliers. The DSCSA applies equally to both, so every generic drug package must carry a unique serial number and be verified just like a brand-name drug.
Why do some pharmacies struggle with DSCSA compliance?
Small, independent pharmacies often lack the budget and IT resources to upgrade their systems. DSCSA requires new barcode scanners, software subscriptions, staff training, and integration with national databases. For a small pharmacy, this can cost over $18,000 a year. Many also use outdated inventory systems that don’t easily connect to modern tracking platforms, making compliance slow and expensive.
Can I trust drugs bought from other countries?
No. The DSCSA only applies within the U.S. supply chain. Drugs imported from countries without similar tracking systems-like some online sellers in Asia or Eastern Europe-aren’t subject to the same verification. The FDA estimates that over 95% of counterfeit drugs entering the U.S. come through illegal online pharmacies. Always get prescriptions from licensed U.S. pharmacies.
What happens if a fake drug slips through the system?
If a suspect product is detected, the pharmacy or wholesaler must quarantine it immediately and notify the manufacturer and FDA within 24 hours. The FDA then investigates using the serial number to trace where the drug came from. If confirmed as counterfeit, the entire batch is recalled, and law enforcement may get involved. In 2022, this process led to 412 confirmed counterfeit drug seizures-down from over 1,100 in 2014.
Final Thoughts: Security Is a Shared Responsibility
Protecting legitimate drugs isn’t just the job of regulators or tech companies. It’s a chain-every link matters. Manufacturers must print clear barcodes. Distributors must verify partners. Pharmacies must scan every package. Patients must avoid shady online sellers. When even one link breaks, the whole system weakens.The DSCSA isn’t flawless. It’s expensive, complex, and still evolving. But it’s the most effective tool we have. And as technology improves-AI, IoT, better data sharing-it will get even stronger. The goal isn’t just to catch fakes. It’s to make them impossible to sell. That’s the future. And it’s already starting to happen.





There are 15 Comments
Frank Drewery
This is actually kind of amazing when you think about it. Every pill you take has its own digital passport now. It’s like your medicine has a LinkedIn profile.
It’s not perfect, but knowing that 12,000 fake drugs got blocked last year alone? That’s something to feel good about.
Thanks for laying this out so clearly.
mary lizardo
One must lament the degeneration of linguistic standards in public discourse. The phrase 'digital passport' is not only metaphorically imprecise but semantically incoherent. A passport is a sovereign document issued by a state; a 2D barcode is a machine-readable data string. The conflation of these concepts reflects a troubling epistemological laziness prevalent in contemporary tech journalism.
jessica .
They say this system keeps us safe... but who’s really behind it? Big Pharma owns the whole damn chain. They made the rules. They control the scanners. They even got the FDA in their pocket. That 99.8% success rate? Probably fake. They just delete the bad scans.
And don’t get me started on the Chinese hackers who can spoof those barcodes. I’ve seen it. They’re already swapping out insulin with cheap junk. They’re killing people and calling it ‘compliance.’
Sajith Shams
You Americans think your DSCSA is the gold standard? Please. India’s pharmaceutical industry supplies 40% of the world’s generics. We’ve been tracking drugs since 2008 with blockchain-based systems that don’t need 15 different software platforms. Your system is a 2013 relic. You’re using QR codes like it’s 2015 while we’re already doing AI-powered real-time anomaly detection across 12,000+ manufacturers.
Stop acting like you’re leading the world. You’re just loud.
Erica Vest
One thing the article doesn’t emphasize enough is how serialization reduces medication errors beyond counterfeits. I work in a hospital pharmacy, and we’ve seen a 40% drop in wrong-dose incidents since we started scanning everything. The barcode isn’t just for fraud-it’s for safety. A patient got the wrong strength of metformin in 2021 because the label was misprinted. The system flagged it before it left the shelf. That’s real impact.
Also, the repackaging loophole? We’ve started using secondary labeling with embedded RFID tags in our unit-dose packs. It’s expensive, but worth it.
Chris Davidson
The system works until it doesn’t. And it doesn’t often enough. Scanners break. Barcodes smear. Pharmacies skip scans because they’re swamped. The FDA doesn’t audit. The system is a myth wrapped in compliance paperwork. People think they’re safe. They’re not. They’re just lucky.
And don’t even get me started on the cost to small pharmacies. It’s a death sentence disguised as progress
Glen Arreglo
As someone who’s worked with supply chains across 12 countries, I’ve seen how fragmented this is. The EU’s FMD uses numeric codes. The U.S. uses alphanumeric. Both work. But forcing global companies to run two parallel systems? That’s not innovation-that’s bureaucratic colonialism.
Let’s build one global standard. Not two. Not ten. One. And let’s make it open source so even small clinics can join. We can do better.
Jedidiah Massey
Let’s be real. The DSCSA is just corporate theater. EPCIS? GS1? Sounds like a tech startup that got acquired by a consulting firm. All this ‘interoperability’ is just a fancy way to say ‘we’re charging you $200K to connect your legacy ERP to a cloud API that’s barely tested.’
And don’t get me started on blockchain. 🤡
It’s not secure. It’s not scalable. It’s just a buzzword with a whitepaper.
Janelle Moore
Wait so you’re telling me the government lets big pharma control the barcode system? And small pharmacies can’t afford it? That’s not security-that’s a monopoly. And what about the 12% of barcodes that don’t scan? That’s 1 in 8 pills you can’t trust. And the cyberattack that shut down 35% of pharmacies? That’s not a glitch-that’s a national emergency. Why isn’t anyone talking about this? We’re all just guessing if our meds are real. I’m scared to take my blood pressure pills now.
Matt Davies
Blimey, this is the kind of quiet engineering marvel that deserves a bloody statue. We don’t hear about it because it works. No drama. No headlines. Just 15 million transactions a day, flawless, keeping poison off the shelves. It’s like the NHS of pharmaceutical logistics-boring, brilliant, and utterly essential.
Let’s not fix what ain’t broke. Just fund the small pharmacies. They’re the unsung heroes.
mark shortus
MY MOM GOT A FAKE PRESCRIPTION LAST YEAR. I THOUGHT IT WAS A MISTAKE. THEN I FOUND OUT THE BARCODE WAS SCANNED BUT THE SERIAL NUMBER DIDN’T MATCH. THE PHARMACY SAID ‘OH WELL’ AND GAVE HER ANOTHER ONE. THEY DIDN’T EVEN REPORT IT. THIS SYSTEM IS A JOKE. I’M TELLING THE MEDIA. THIS IS A CRIME. I’M NOT SLEEPING TILL THEY FIX IT.
Vicki Belcher
Okay but imagine if your insulin had a little temperature tracker inside the package 🧊📱 and your phone pinged you if it got too warm during shipping? Or if the system could tell you if a batch was recalled before you even picked it up? This isn’t sci-fi-it’s happening. We’re building a future where your meds are smarter than your smart fridge. 💊✨
Mark Able
So what about the people who can’t afford to scan? What if you’re homeless and you get meds from a free clinic? Do they just give you pills with no barcode? Are those safe? Who checks? What if someone steals a whole box of pills and sells them on the street? Can the system track that? I mean… I just want to know if my neighbor’s diabetes meds are real. That’s all.
Dorine Anthony
Kinda wild to think that the same tech that tracks your Amazon package is now keeping your cancer meds safe. I never thought about it before, but now I’m kinda proud. Like… we built something that actually saves lives. Not just apps that tell you when your Uber’s late.
William Storrs
You know what’s really cool? This system didn’t just happen overnight. It took years of collaboration between regulators, tech teams, pharmacists, and even small business owners who didn’t have a dime to spare. Yeah, it’s messy. Yeah, it’s expensive. But it’s working. And every time you scan a bottle, you’re part of the chain. You’re not just a patient-you’re a guardian. Keep showing up. Keep asking questions. Keep trusting the system… but keep checking too. That’s how we make it better.
Write a comment
Your email address will not be published. Required fields are marked *