
Common Variable Immunodeficiency isn't just a rare disease-it's a hidden battle many people fight for years before getting answers. Imagine getting sick every few weeks with sinus infections, pneumonia, or stomach bugs that won’t go away. Antibiotics help for a while, but the infections keep coming back. You feel tired all the time. You lose weight even when eating well. Doctors tell you it’s allergies or stress. Then, after eight years and five different doctors, someone finally says: you have CVID.
CVID is a primary immunodeficiency where your body doesn’t make enough antibodies. Antibodies are the proteins your immune system uses to recognize and kill germs like bacteria and viruses. Without them, even common infections become dangerous. The problem isn’t that you don’t have immune cells-it’s that your B cells, which should turn into antibody factories, just don’t work right. You might have plenty of B cells floating around, but they’re like workers without tools. They show up to the job but can’t build what’s needed.
What Makes CVID Different From Other Immune Disorders?
People often confuse CVID with other immune problems. Selective IgA Deficiency, for example, only affects one type of antibody-IgA. Many people with that condition never even know they have it. CVID is worse because it hits multiple antibodies at once. IgG, the most important one for fighting infections, drops below 500 mg/dL-sometimes as low as 100 mg/dL. Normal levels are 700-1600. IgA, which protects your lungs and gut, often disappears completely. IgM, another key player, is also low in half the cases.
Compare that to X-linked agammaglobulinemia (XLA), another antibody disorder. In XLA, kids are born with almost no B cells at all. They get sick as babies. CVID usually shows up in adults between 20 and 40. That delay is why so many people go undiagnosed for years. By the time they’re diagnosed, they’ve already suffered lung damage from repeated pneumonia, or developed chronic gut inflammation from parasites like Giardia.
Why Is Diagnosis So Hard?
There’s no single blood test that says, “You have CVID.” Diagnosis requires three things: low antibody levels, poor response to vaccines, and ruling out other causes. Doctors check IgG, IgA, and IgM levels. Then they give you a vaccine-like pneumococcal or tetanus-and test your blood again after a few weeks. If your antibody levels don’t rise, that’s a red flag.
But here’s the catch: 80-85% of CVID cases have no known genetic cause. Even with advanced DNA testing, scientists can’t pinpoint why the B cells fail in most patients. That makes it hard to predict who will get lung disease, who will develop autoimmune problems, or who will be at risk for lymphoma. Some people only get colds. Others end up with Crohn’s-like bowel disease or spleen enlargement. One person might need treatment every two weeks. Another might go months without an infection.
Patients report spending an average of 8.2 years seeing doctors before getting the right diagnosis. That’s longer than most people spend in college. During that time, they’re treated for asthma, allergies, chronic bronchitis, or even depression. Fatigue gets labeled as burnout. Weight loss gets called “metabolic issues.” The truth? Their immune system is broken.

How Is CVID Treated Today?
There’s no cure. But there is a treatment that changes everything: immunoglobulin replacement therapy.
This therapy gives you back the antibodies your body can’t make. It’s made from plasma-the liquid part of blood-from thousands of healthy donors. The plasma is purified, filtered, and turned into a solution you get through an IV or under your skin.
There are two main ways to get it:
- Intravenous Immunoglobulin (IVIG): You go to a clinic every 3-4 weeks. A nurse hooks you up to an IV. The infusion takes 2-4 hours. You might get headaches, chills, or nausea during or after. About 32% of patients report these reactions.
- Subcutaneous Immunoglobulin (SCIG): You inject it under your skin, usually once a week. You can do it at home after a few training sessions. Most people master it within two months. Side effects are usually just redness or swelling at the injection site.
Doctors aim to keep your IgG level above 800 mg/dL. That’s the sweet spot where infections drop off dramatically. Studies show patients on consistent therapy go from 10-12 infections a year down to 2-3. Energy levels improve. Weight gain becomes possible. Many people return to work, travel, and even have kids.
Cost is a major barrier. In the U.S., IVIG runs $65,000-$95,000 a year. SCIG is slightly more expensive, $70,000-$100,000. Insurance covers most of it, but co-pays and deductibles still hurt. In low-income countries, only 35% of diagnosed patients get treatment at all. That’s not just a medical issue-it’s a global inequality problem.
What Happens If You Don’t Treat It?
Left untreated, CVID doesn’t just mean more colds. It means irreversible damage.
Chronic lung infections lead to bronchiectasis-permanent widening of the airways. Mucus builds up. Bacteria grow. Breathing becomes harder. By age 50, 65% of untreated CVID patients have this condition. Only 15% of healthy people do.
Then there’s the gut. About 40% of patients develop inflammation in their intestines. Some get chronic diarrhea from Giardia. Others develop a condition called granulomatous disease-clumps of immune cells that damage organs. It looks like sarcoidosis or Crohn’s, but it’s caused by the same immune dysfunction.
And then there’s cancer. People with CVID have a 20-50 times higher risk of lymphoma than the general population. That’s not a small increase. It’s a life-altering risk. That’s why regular scans and blood work aren’t optional-they’re essential.
Autoimmune problems are also common. About 25% of CVID patients develop conditions like immune thrombocytopenia (ITP), where the body attacks its own platelets. Others get autoimmune hemolytic anemia, where red blood cells are destroyed. Rheumatoid arthritis shows up too. These aren’t side effects-they’re part of the disease.
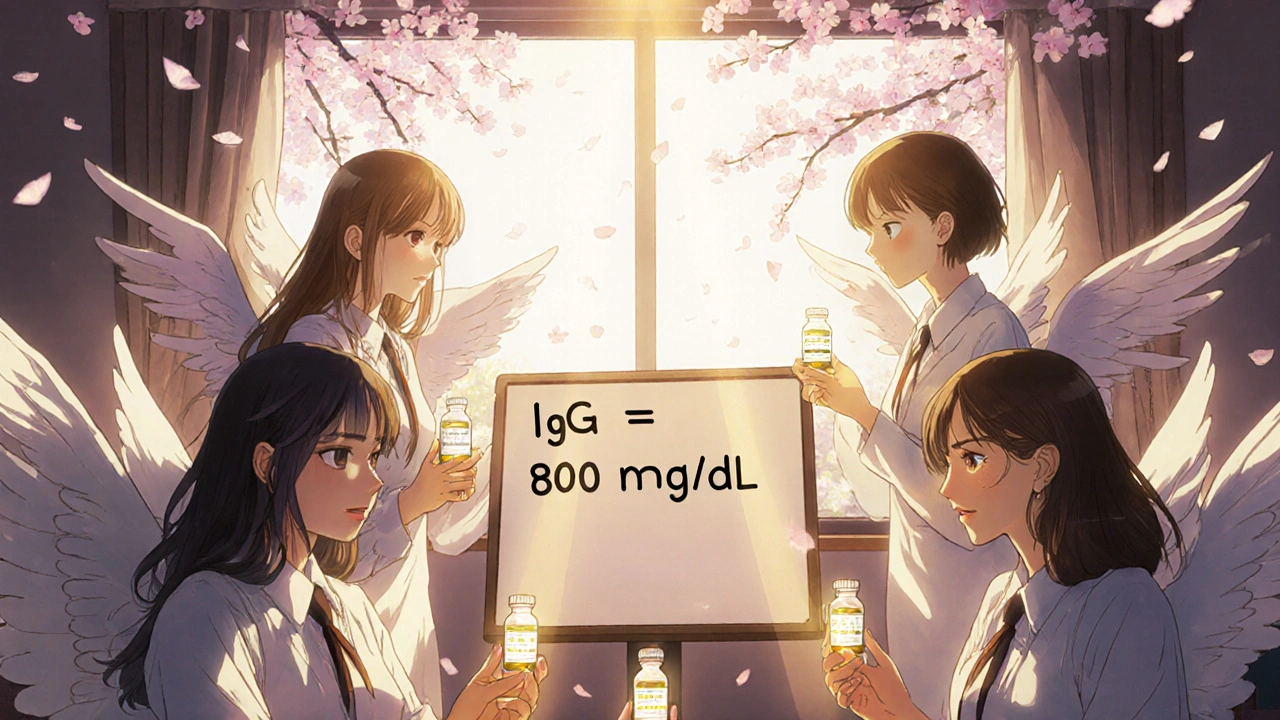
What’s Next for CVID Treatment?
The future is starting to look brighter. Researchers are no longer treating CVID as one disease. They’re breaking it into subtypes based on genetics and symptoms. Some patients have mutations in the TACI gene. Others have problems with BAFF-R. These differences might explain why some respond better to certain drugs.
One promising drug, atacicept, is in late-stage trials. It targets two proteins-BAFF and APRIL-that overstimulate bad immune cells. In early tests, it cut severe infections by 37% compared to immunoglobulin alone. It’s not a replacement yet, but it could be a powerful add-on for patients who still get sick despite treatment.
Another hope is recombinant antibodies-lab-made versions that don’t need human plasma. Right now, the entire supply chain depends on blood donations. But plasma shortages are growing. The industry reports a 12% gap between what’s needed and what’s available. That means prices could rise 15-20% a year. Recombinant products could solve that.
Dr. Sergio Rosenzweig of the NIH predicts that within five years, we’ll be able to test a CVID patient’s DNA and say, “You’re in subtype 3. You’ll respond best to X drug.” That’s the future: personalized medicine for immune disorders.
Living With CVID: What Patients Say
People with CVID aren’t just patients-they’re survivors. On patient forums, they share real stories:
- “I used to miss work every other month. Now, with SCIG at home, I’ve been to only one doctor’s appointment in six months.”
- “I lost 30 pounds because I couldn’t absorb food. After starting treatment, I gained it back. I can eat pasta again.”
- “My kids used to be scared to hug me. Now I can take them to the park without worrying I’ll get sick and end up in the hospital.”
But it’s not all good. Some say the cost is crushing. Others say nurses don’t know how to help with home infusions. Some get dismissed by doctors who’ve never seen a case. Support groups-like the Immune Deficiency Foundation-are lifelines. They host 200+ local chapters and a 2,500-person annual conference. That’s where people learn how to advocate for themselves.
Life expectancy has doubled since the 1980s. Back then, most CVID patients died by age 33. Now, with consistent treatment, the median survival is 59. That’s not a cure. But it’s progress. It’s hope.
Is CVID the same as having low immunity from stress or poor diet?
No. CVID is a genetic disorder where your body can’t make antibodies, no matter how healthy you are. Stress or bad diet can make you more prone to infections, but they don’t cause IgG levels to drop below 500 mg/dL. CVID requires lab testing to diagnose-it’s not something you can fix with vitamins or sleep.
Can you outgrow CVID?
No. CVID is a lifelong condition. The immune system doesn’t fix itself. Even if symptoms improve for a while, the underlying antibody deficiency remains. Stopping treatment leads to a return of infections and complications. Lifelong immunoglobulin therapy is the standard.
Is CVID contagious?
No. CVID is not an infection. You can’t catch it from someone else. It’s caused by genetic changes, not viruses or bacteria. People with CVID are more likely to get infections, but they can’t pass CVID on to others.
Do all CVID patients need immunoglobulin therapy?
Almost all do. A small number with very mild symptoms might delay treatment, but doctors strongly recommend starting therapy as soon as diagnosis is confirmed. Without it, the risk of permanent lung damage, autoimmune disease, and cancer rises sharply. Waiting is dangerous.
Can you get vaccinated if you have CVID?
Yes-but not all vaccines work. Live vaccines (like MMR or chickenpox) are unsafe because your immune system can’t control them. Inactivated vaccines (like flu shots or tetanus) are safe and often given to test your response. But since your body can’t make antibodies, these vaccines won’t protect you. That’s why immunoglobulin therapy is still needed.
What’s the difference between IVIG and SCIG?
IVIG is given through a vein in a clinic every 3-4 weeks. It takes hours and can cause side effects like headaches or chills. SCIG is injected under the skin, usually weekly, at home. It’s slower but gentler. Most people prefer SCIG for convenience and fewer side effects. Both deliver the same antibodies-just different delivery methods.





There are 8 Comments
Robert Bashaw
This isn't just a medical condition-it's a slow-motion horror movie where the villain is your own body. Imagine your immune system being a car with a full tank of gas but no engine. You can press the pedal all day, but you're not going anywhere. And then, after years of being told you're just 'stressed out' or 'a hypochondriac,' someone finally says, 'Your B cells are just standing around like they're on a coffee break.' That moment? Pure relief. And then the bills hit. $90k a year just to keep from coughing up a lung. I don't know if I'm more angry at the disease or the insurance company.
Brandy Johnson
It is regrettable that the narrative presented here lacks sufficient empirical grounding in public health policy. The assertion that immunoglobulin therapy is universally accessible is demonstrably false within the context of global resource allocation. Moreover, the romanticization of patient narratives without corresponding data on long-term mortality reduction constitutes a form of medical sensationalism. The United States spends more per capita on healthcare than any other nation, yet CVID remains underdiagnosed. This is not a failure of biology-it is a failure of systemic prioritization.
Peter Axelberg
I’ve been living with this for 14 years. I used to think I was just unlucky-always catching everything, always tired, always losing weight even when I was eating like a bear before hibernation. Then I got diagnosed. SCIG changed everything. I do it myself now, right on the couch while watching Netflix. No more clinics. No more IV lines that feel like a bad dream. My kids don’t tiptoe around me anymore. I took them to Disney last year. I didn’t get sick. I didn’t miss a day of work. That’s not a treatment-that’s a second life. And yeah, it’s expensive. But what’s the cost of never seeing your kid’s first soccer game? I’d pay double.
Monica Lindsey
People don’t understand that CVID isn’t ‘just’ a disease. It’s a lifestyle surrender. And the fact that you’re still alive at 59? That’s not progress. That’s barely survival. Stop calling it hope. Call it damage control.
jamie sigler
I just feel so bad for these people. Like, imagine knowing your body is broken and you can’t fix it. And then you have to pay for the band-aid. It’s just… heavy. I don’t even know what to say. I guess I’ll just sit here and cry quietly.
Bernie Terrien
They’re calling it a ‘disease’ but it’s really just a corporate cash cow wrapped in medical jargon. Plasma isn’t magic-it’s blood from donors who get paid $50 a pop. Meanwhile, the same companies charge $100k/year to keep you alive. And don’t get me started on ‘recombinant antibodies’-that’s just Big Pharma’s next gold mine. They’ll sell you a $200k/year vial labeled ‘innovation’ while the real solution is cheaper, simpler, and ignored.
Jennifer Wang
It is imperative to clarify that while immunoglobulin replacement therapy remains the standard of care, emerging biomarkers-including B-cell subsets and somatic hypermutation profiles-are increasingly being used to stratify patients into clinically meaningful subtypes. This paradigm shift enables targeted therapeutic interventions beyond antibody replacement, such as BAFF/APRIL inhibition in TACI-deficient cohorts. Furthermore, prophylactic pulmonary function monitoring and early surveillance for lymphoproliferative disorders are now considered essential components of longitudinal management. The data supporting these protocols are robust and peer-reviewed.
stephen idiado
Western medicine fetishizes plasma. In Nigeria, we treat chronic infections with herbal immunomodulators and ancestral protocols. Your ‘antibody deficiency’ is a symptom of systemic toxicity, not genetic failure. Your IVIG is a colonial placebo. The real cure? Detox. Fasting. Spiritual alignment. You’re treating the symptom, not the soul.
Write a comment
Your email address will not be published. Required fields are marked *